The country "opens the green light"! The consistency evaluation of the billions of large varieties
[Hui Cong Pharmaceutical Industry Network] Yesterday, the General Office of the State Council issued the "Opinions of the General Office of the Medical Reform Office of the State Council on Reforming and Perfecting the Supply and Protection Policy of Generic Drugs". This news instantly wiped out the circle of friends. The opinions were clearly and comprehensively implemented. Through the follow-up policy of conformity evaluation products, we provided comprehensive generic drug support from procurement, medical insurance, taxation, publicity, etc., and requested the research and development of generic drugs, and promoted the substitution of generic drugs and original drugs. use.
Up to now, a total of 14 products have passed the consistency evaluation, involving 11 enterprises, Huahai, Jingxin, Xinlitai, etc., among which Huahai has become the biggest winner with 7 varieties and 9 specifications. In 2017, the import value of western medicine in China was 115.84 billion yuan, and the top ten varieties were mostly patent-expired drugs. This led to the layout of pharmaceutical companies, and strived to launch the first shot of the consistency evaluation of generic drugs.
Many initiatives support the imitation of the original research, cracking "big but not strong"
For a long time, China's generic drug production field lacks high-level quality standards and quality control systems, and the whole industry generally operates at low cost. As a result, some of the generic drugs that have been approved for listing are generally of lower quality than the original drugs, and the high-quality drug market is mainly Under the rule of foreign original research drugs, the original research drugs can not only maintain high prices, but also maintain a large market share under the rules of bidding for one product and two regulations. Therefore, many products that have been extremely shrinking in global market sales in the past years have been Can continue to shine in China. According to the import and export data of the Medical Insurance Chamber of Commerce in 2017, the import value of western medicine in China is 17.157 billion US dollars, accounting for 115.84 billion yuan, and the top ten varieties are mostly patent-expired drugs.
Vigorously developing high-quality generic drugs is an inevitable choice for pharmaceutical powers. It can effectively reduce the cost of medicines, improve the accessibility and affordability of medicines, and thus ensure the sustainable development of medical insurance funds. In order to improve the situation of generic drugs in China, the State Council has issued a policy document encouraging the development of generic drugs. The biggest highlight of this document is its internal and external repairs, from clinical to industrial, from domestic to foreign, from procurement and payment. , comprehensive guidance on clinical guidelines, intellectual property, and publicity. So how do you judge whether the generic drug is of high quality? The biggest criterion is to pass the “similar evaluation of generic drugsâ€. Up to now, a total of two batches of products have passed the “consistency evaluation†(involving 14 products).
14 consistent evaluation varieties, 10 sales broke 1 billion
Table 1:14 Evaluation of drugs that have passed the consistency of generic drug quality and efficacy
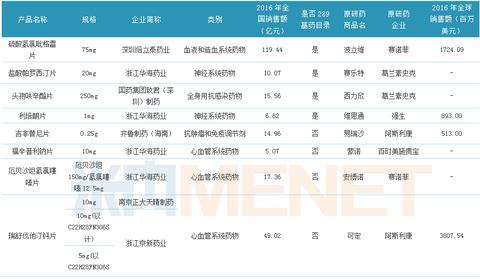
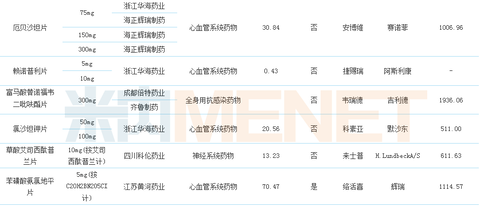
(Source: CFDA official website, Mene network database)
Up to now, CFDA has released two batches of products that have passed the quality and efficacy consistency evaluation of generic drugs, involving 14 products, of which 5 289 drug-based catalogues account for about 1/3. According to the regulations, the consistency evaluation of the 289 basic drug list varieties will be completed by the end of 2018, while the non-289 drug list varieties have no time regulations. In contrast, the consistency evaluation conditions of the 289 base drug list seem to be more demanding. Therefore, the pharmaceutical companies have focused their attention on other potential varieties, trying to get the first time to get the consistency evaluation tickets and enjoy more policy dividends.
The 14 products that have passed the consistency evaluation involve 11 enterprises. Among them, Huahai Pharmaceutical has become the biggest winner with 7 varieties and 9 acceptance numbers. Haizheng Pfizer has one variety and three acceptance numbers. Zhejiang Jingxin Pharmaceutical Co., Ltd. has one variety, two acceptance numbers ranked third, and the remaining pharmaceutical companies have one variety, and one acceptance number passed the consistency evaluation.
There are 3 specifications for rosuvastatin calcium tablets, and 2 companies have been approved, namely Nanjing Zhengda Tianqing Pharmaceutical and Zhejiang Jingxin Pharmaceutical. Among them, Zhejiang Jingxin Pharmaceutical has 2 specifications approved; irbesartan tablets have Three specifications, two companies were approved, namely Zhejiang Huahai Pharmaceutical and Haizheng Pfizer, among which Haizheng Pfizer has taken all the specifications; tenofovir disoproxil fumarate tablets have one specification, 2 The companies were approved, namely Chengdu Beite Pharmaceutical and Qilu Pharmaceutical.
14 products cover blood and hematopoietic system, nervous system, systemic anti-infective, anti-tumor and immunomodulator, cardiovascular system, etc. Seven of them belong to cardiovascular system drugs, which are 50% ahead of others. Category products, there are 3 drugs in the nervous system, and 2 anti-infective drugs for systemic use, ranking second and third respectively.
Figure 1: Sales of products with sales exceeding 1 billion yuan in consistency evaluation (unit: 100 million yuan)
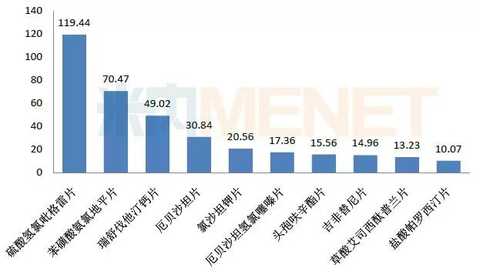
(Source: Minenet database)
According to the data of the intranet, 14 of the products that have passed the consistency evaluation, the 2016 China City Public Hospital, the county-level public hospital, the urban community center, and the township hospital (referred to as the Chinese public medical institution) terminal and the Chinese city retail pharmacy terminal total sales There are 1 product with a total amount of more than 10 billion yuan, which is the clopidogrel sulfate tablets of Shenzhen Xinlitai. The total sales of more than 1 billion yuan (including 10 billion varieties) are 10 pieces of paroxetine hydrochloride tablets. Cefuroxime axetil tablets, gefitinib tablets, irbesartan hydrochlorothiazide tablets, rosuvastatin calcium tablets, irbesartan tablets, losartan potassium tablets, escitalopram oxalate tablets, ammonia benzenesulfonate Clodipine tablets.
Clopidogrel: three legs, the original research occupied half of the country
Figure 2: The pattern of clopidogrel in China's public medical institutions and Chinese urban retail pharmacies in 2016
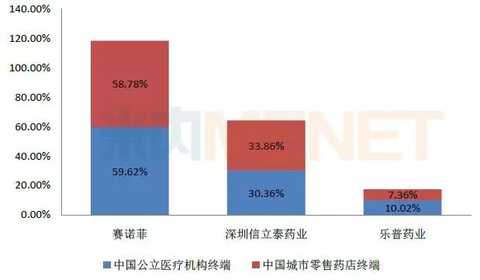
(Source: Minenet database)
Clopidogrel, a classic anticoagulant in the cardiovascular and cerebrovascular fields, according to the data from the intranet, the sales of clopidogrel in China's public medical institutions and Chinese urban retail pharmacies in 2016 were 10.084 billion yuan and 1.86 billion yuan respectively, totaling 119.44. 100 million yuan.
At present, only three companies in China produce clopidogrel, which is Sanofi, Xinlitai and Lepu Pharmaceuticals, presenting a situation of “three pillarsâ€. In China's urban public medical institutions and China's urban retail pharmacy terminals, Sanofi, as the original manufacturer, has a dominant position in the overall market of clopidogrel. With the consistent evaluation of Xinlitai's 75mg clopidogrel, its market share It is expected to increase substantially.
Amlodipine: Huanghe Pharmaceutical seizes the opportunity, the original research accounted for 60.99%
Figure 3: The amlodipine pattern in China's public medical institutions in 2016
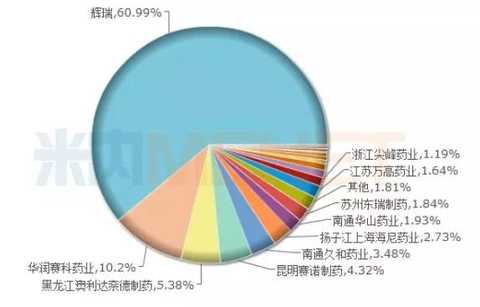
(Source: Minernet China's public medical institutions terminal competition pattern)
Amlodipine, a calcium ion antagonist, can protect the kidneys when used alone or in combination with other drugs to treat high blood pressure and angina pectoris. According to the data from the intranet, the sales of amlodipine in China's public medical institutions and China's urban retail pharmacies in 2016 were 5.227 billion yuan and 1.82 billion yuan respectively, with a total sales of 7.047 billion yuan.
In addition to the original researcher Pfizer occupying a major market share, the share of other pharmaceutical companies is not much different, and the competition is fierce. In 2016, the amlodipine besylate tablets of Jiangsu Huanghe Pharmaceutical Co., Ltd. have not yet entered the TOP20 brand of amlodipine, but as the only 289-based drug list that has passed the consistency evaluation, there will be no similar products in the next three years. Accepted, the amlodipine of the Yellow River Pharmaceuticals seized the opportunity, I believe that will soon be able to share a cup of glutinous rice.
Rosuvastatin: the proportion of the original research decreased year by year, Jingxin, Zhengda Tianqing grabs food
Figure 4: The rosuvastatin pattern in the terminal of Chinese public medical institutions in 2016
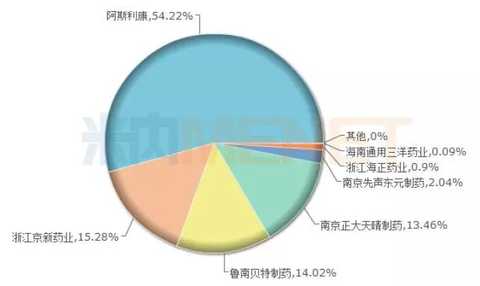
(Source: Minernet China's public medical institutions terminal competition pattern)
Rutstatin calcium has high selectivity to the liver, and the lipid-lowering effect is more obvious, and the side effects on the central nervous system are lower than other statins. According to the data from the intranet, in 2016, the sales of rosuvastatin calcium tablets in China's public medical institutions reached 4.206 billion yuan, and the sales of retail pharmacies in China reached 696 million yuan. In recent years, AstraZeneca's market share has been declining, and 63.5% in 2014 fell to 55.47% in 2016.
According to the Intranet Global Drug Research and Development Library, AstraZeneca's rostastatin calcium has global sales of US$3807.5 million in 2016. Nanjing Zhengda Tianqing Pharmaceutical and Jingxin Pharmaceutical's rosuvastatin calcium tablets passed the consistency. Evaluation, the "big cake" ranks among the people.
Imported pharmaceutical brand TOP10, mostly patent expired products
Figure 5: 2013-2016 China's public medical institutions terminal imported chemical brand TOP10
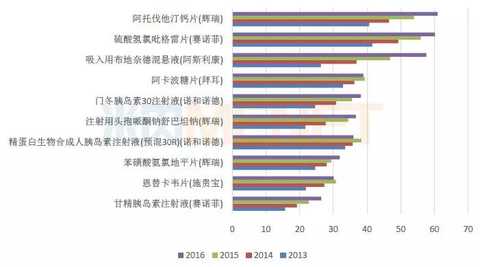
(Source: Minernet China's public medical institutions terminal competition pattern)
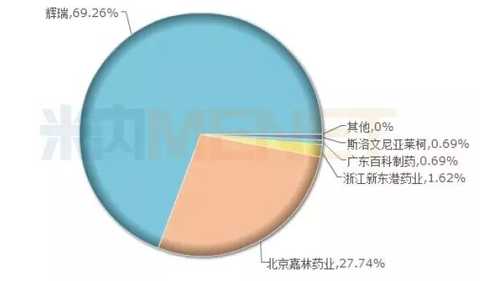
Atorvastatin calcium tablets: the road of domestic brands
Atorvastatin calcium tablets (Lipitor) is a generation of "medicine". Since its inception, the company has earned hundreds of billions of dollars for its development company Pfizer, becoming the global drug sales myth. With the expiration of patent protection in the global pharmaceutical market in May 2012, the global sales of the drug showed a cliff-like decline, and the market shrank severely. However, in China, it still maintained a good market growth rate and has been at the forefront of imported brands for many years. In 2016, the sales volume of Liputo domestic public hospitals reached US$6.086 billion, accounting for 69.26% of the market share, while the second-ranked domestic brand Beijing Jialin’s “Ale†only occupied 27.74%, which is quite different. According to the MED China Drug Evaluation Database 2.0, Beijing Jialin Pharmaceutical and Zhejiang Xindonggang Pharmaceuticals have carried out the consistency evaluation of atorvastatin calcium tablets. It is believed that after the domestic brands complete the consistency evaluation, Pfizer's market The share will see a huge decline in the next few years, and a single big will eventually become history.
Figure 6: 2016 atorvastatin calcium tablet pattern in public medical institutions in China
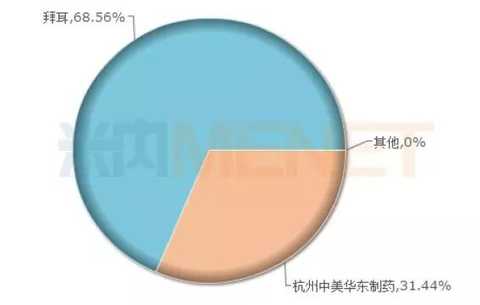
Acarbose tablets: domestic leader - Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd.
Developed by Bayer, Acarbose was first marketed in Switzerland in 1975 and is the world's first approved alpha-glucosidase inhibitor. Due to its excellent hypoglycemic effect, the drug has been recommended as a first-line treatment for type 2 diabetes. The global patent for acarbose has long expired, but only Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd., which has obtained the approval of Acarbose tablets in China. In 2016, Bayer Acarbose (Bai Tang Ping) domestic public medical institutions market sales of 3.887 billion, accounting for 68.56% of the share, Hangzhou Zhongmei Huadong Pharmaceutical accounted for 31.44%. At present, Hangzhou Sino-US Huadong Pharmaceutical is actively conducting a consistent evaluation, and there are many pharmaceutical companies in the country to lay out the imitation of this drug. This huge hypoglycemic market has attracted more intense competition.
Figure 7: 2016 Acapoose Tablet Pattern in China's Public Medical Institutions
Clopidogrel Hydrochloride Tablets: Xinlitai will eventually "counterattack"
Clopidogrel is a new generation of anticoagulant developed by Sanofi and Bristol-Myers Squibb. Clopidogrel was first launched in the United States in 1997. With the advantages of good efficacy and low adverse reactions, the sales in the fourth year of the market are close. With $2 billion, global sales in 2011 were close to $10 billion. However, since the expiration of the US core compound patent on May 17, 2012, sales have fallen. There are currently three companies in the Chinese market that produce clopidogrel formulations. Xinlitai's Taijia (clopidogrel hydrogen sulfate tablets) was approved for listing in 2000. The original research drug Polivi was listed in 2001 after obtaining administrative protection. Due to certain historical reasons, Taijia was able to coexist with the original drug in the Chinese market for a long time. The second domestic clopidogrel did not enter the market until 2012. Sanofi's Polivi has been in a dominant position in the domestic market for many years. In 2016, the domestic market share was 59.62%, Xinlitai's Taijia was 30.36%, and Lepu Pharmaceuticals accounted for 10.01%. In the first batch of published consensus evaluation lists, Xinlitai's Taijia is in the list, and Lepu Pharmaceutical is also actively carrying out the consistency evaluation, and it is inevitable that domestic brands will gain a larger market share.
Inhaled budesonide suspension and insulin aspart 30 injection
Inhaled budesonide suspension and insulin aspart 30 injection are exclusive products. At present, only foreign companies sell in China, and the market share in 2016 is the third and fifth place respectively in imported brands. According to the MED China Drug Evaluation Database 2.0, the current domestic imitation of budesonide suspension for inhalation is only two, and it is still a long time away from the market. It can be seen that AstraZeneca will remain in the dominance of the budesonide drug market for a long time. Insulin Insulin 30 injection is currently only produced and sold by Novo Nordisk, accounting for an absolute proportion. However, the Zhuhai Federation submitted the application for the insulin aspart 30 injection in January 2018, and it is expected to be available this year. At the same time, the research and development progress of Gandong Pharmaceutical Insulin 30 injection is also among the top enterprises in China. It is expected that the product will be before 2019. Listing. In the future, inhaled budesonide suspension and insulin aspart 30 injection market, domestic brands are difficult to incite the status of the original research drug.
Editor in charge: Chen Wei
Advantages:
1.Design team: We have a professional design team. Over 5000 designs for selection.
2.Rapid response to your needs: Welcome to contact with us if you have any problems.
4.Efficiency: Our 50 designers will keep you updated with 15 new items each month.
5.Professional factory: We are the biggest manufacturer of table cloth in China,competitive price with good quality.
3.Standard: All of our models are approved by ISO9001, SGS and Testex.
| Product Name | Eco Friendly Bath Car Kitchen Drawer Bathroom Yoga Rubber Anti-Slip Anti Slip Mat |
| Shape | Rectangle |
| Material | PVC foam with polyester net. |
| Thickness | 1mm ~ 5mm / custom size. |
| Width | <1.47M (58 Inch) |
| Color | Solid or printed. As your request. |
| Size |
65CM x 15M / Roll, custom. Roll and Sheet. |
| Usage | widely used |
| Feature | Non slip, Soft, High quality, Waterproof, Oilproof |
Kitchen Mats,Weathertech Floor Liners,Floor Mats For Home,Welcome Mat
IUIU Household Co.,Ltd , https://www.iuiupvcgroup.com